Is NF3 Polar? Unpacking The Chemistry Of Nitrogen Trifluoride Today
Have you ever stopped to think about how tiny molecules, the building blocks of everything around us, have their own distinct personalities? It's really quite something, you know, how their shapes and the way their electrons are arranged can make all the difference. Sometimes, these small differences can lead to big impacts on how a substance behaves, whether it mixes with water, or how it acts in different situations.
It's almost like a puzzle, putting together the pieces of information to see the full picture of a molecule. We look at what atoms are present, how they connect, and what that means for the overall electron distribution. This process helps us figure out why one substance might be a gas at room temperature while another, with a similar makeup, is a liquid, or even a solid, you see.
Today, we're going to take a closer look at a specific molecule, NF3, which is nitrogen trifluoride. People often wonder about its properties, especially if is NF3 polar, and that's a very good question to ask. We'll explore what makes a molecule have a "charge separation" and then apply those ideas to this particular compound, so it's quite an interesting topic.
Table of Contents
- What Exactly is NF3?
- Getting to Grips with Molecular Polarity
- The Idea of Electronegativity
- Understanding Bond Polarity
- The Role of Molecular Shape
- Putting it All Together: Is NF3 Polar?
- Looking at the N-F Bonds
- Considering the Nitrogen Atom's Lone Pair
- Visualizing the Shape of NF3
- Why Does NF3's Polarity Matter?
- Frequently Asked Questions About Polarity
- What makes a molecule polar?
- How can you tell if a molecule is polar?
- Are all molecules with polar bonds also polar molecules?
- What to Explore Next in Chemistry
What Exactly is NF3?
Let's start with the basics, shall we? NF3 is a covalent compound, and that's important to remember. It consists of one nitrogen atom joined up with three fluorine atoms, you know, forming a specific structure. These atoms share electrons to create those connections, which is what "covalent" means in this context.
This molecule, nitrogen trifluoride, is a gas at room temperature, actually. It has some uses in the electronics industry, for example, in making computer chips. So, while it might seem like just a chemical formula, it has a role in technology, too.
The way these atoms come together, the nitrogen and the three fluorines, sets the stage for everything else we'll discuss about its characteristics. It's a fundamental building block for understanding its behavior, really.
Getting to Grips with Molecular Polarity
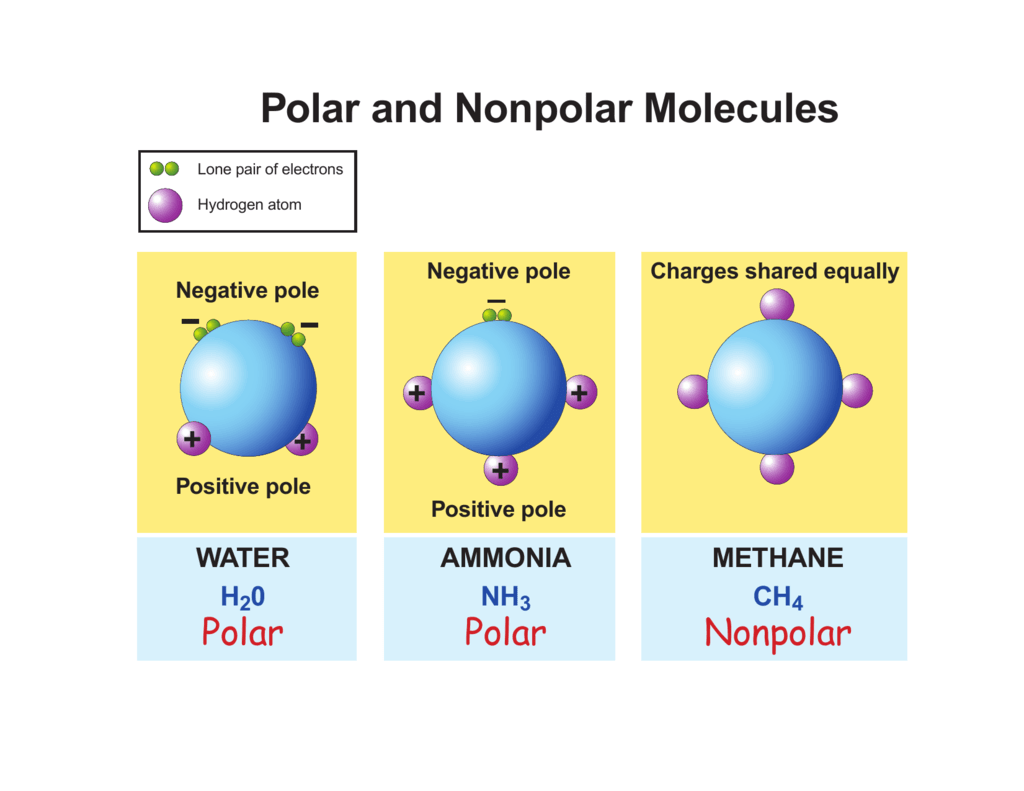
Before we can figure out if NF3 is polar, we need to understand what "polar" even means when we talk about molecules. It's about how electrons are shared, or not shared equally, within the molecule, you see. This unequal sharing can create areas that have a bit of a positive charge and areas that have a bit of a negative charge.
Think of it like a tiny magnet, almost. One end has a certain pull, and the other end has a different pull. Molecules can have this kind of "magnetic" quality, in a way, if their electron distribution isn't perfectly even across the whole structure.
The Idea of Electronegativity
The concept of electronegativity is pretty central to all of this. It's a measure of how strongly an atom pulls on shared electrons in a chemical bond, you know. Different atoms have different strengths when it comes to this pulling power.
Some atoms are real electron hogs, so to speak, pulling those shared electrons much closer to themselves. Others are a bit more relaxed about it. This difference in pulling power is what sets up the stage for polarity, actually.
When two atoms with very different electronegativity values connect, the electrons they share aren't sitting exactly in the middle. They spend more time closer to the atom that has the stronger pull, you see. This creates an imbalance, and that's the first step towards a polar situation.
Understanding Bond Polarity
So, because of electronegativity differences, individual connections between atoms can become "polar bonds." This means that within that one connection, one atom gets a slight negative charge because the electrons are hanging around it more often, and the other atom gets a slight positive charge, you know.
It's like a tug-of-war where one side is clearly stronger. The rope, representing the shared electrons, gets pulled closer to the stronger team. That's a polar bond, basically.
Not all connections between atoms are polar, though. If two atoms have the same, or very similar, pulling power, then the electrons are shared pretty evenly. Those would be called nonpolar bonds, in that case.
The Role of Molecular Shape
Now, this is where things get a little more interesting, perhaps. Even if a molecule has polar bonds, the whole molecule might not be polar. Why is that, you ask? It all comes down to the molecule's overall shape, you see.
Imagine those individual bond polarities as little arrows, each pointing towards the atom that's pulling the electrons more strongly. If these arrows are arranged in a way that they all cancel each other out, then the molecule, as a whole, ends up being nonpolar. It's like having two equal and opposite forces pulling on something, and it just stays put, you know.
But if those arrows don't cancel out, if there's an overall direction to the electron pull, then the molecule will be polar. The shape determines if there's a net "charge separation" across the entire molecule, actually. This is a very important concept to grasp when thinking about polarity.
Putting it All Together: Is NF3 Polar?
Alright, so with all that in mind, let's finally tackle the big question: is NF3 polar? The answer is yes, it is a polar molecule. And we can figure out why by looking at its bonds and its shape, too.
Looking at the N-F Bonds
First, let's consider the individual connections between nitrogen and fluorine. Fluorine is one of the most electron-hungry atoms out there, you know, it has a very strong pull. Nitrogen, while also having a good pull, isn't quite as strong as fluorine.
Because of this difference in electron-pulling power, each nitrogen-fluorine bond in NF3 is, in fact, a polar bond. The electrons in each connection spend more time closer to the fluorine atom. So, each fluorine atom gets a slight negative charge, and the nitrogen atom gets a slight positive charge, as a matter of fact.
So, we've established that the individual connections are polar. That's a good start, but it doesn't automatically mean the whole molecule is polar, as we learned earlier. We need to look at the bigger picture, too.
Considering the Nitrogen Atom's Lone Pair
This is a crucial detail for NF3. The nitrogen atom in NF3 has what we call a "lone pair" of electrons, you know. These are electrons that are not involved in forming connections with other atoms. They just sit on the nitrogen atom.
These lone pair electrons take up space around the central nitrogen atom. They push the other connections, the ones to the fluorine atoms, away from themselves. This pushing action influences the overall shape of the molecule, you see, quite significantly.
The presence of this lone pair means the electron distribution around the nitrogen atom isn't perfectly symmetrical. It's like having an extra bulge on one side, which impacts the balance of the whole thing, basically.
Visualizing the Shape of NF3
Because of that lone pair on the nitrogen atom, NF3 doesn't form a flat, symmetrical shape. Instead, it takes on what's called a trigonal pyramidal shape. Imagine a pyramid with the nitrogen atom at the top, and the three fluorine atoms forming the base, you know, like a tripod.
Now, think about those individual polar N-F bonds we talked about. Each one is pulling electrons towards its fluorine end. If the molecule were perfectly flat, or if it had a different, more symmetrical shape, these pulls might cancel each other out, you see.
But because of the trigonal pyramidal shape, the pulls from the three N-F bonds don't cancel. The lone pair on the nitrogen pushes the fluorine atoms downwards, creating an uneven distribution of electron density across the molecule. So, the "negative" ends of the bond dipoles are all pointing somewhat downwards, and they don't have an equal and opposite pull to balance them out, actually.
This means there's a net overall pull of electrons towards one side of the molecule. This creates a distinct positive end and a distinct negative end for the entire NF3 molecule. So, yes, it has a permanent charge separation, making it a polar molecule, you know, quite definitively.
Why Does NF3's Polarity Matter?
Understanding if NF3 is polar isn't just a fun chemistry fact, it has real implications for how the molecule behaves. Polar molecules tend to interact with other polar molecules, you see. This is often described as "like dissolves like."
For example, polar substances usually dissolve well in polar solvents, like water. Nonpolar substances, on the other hand, prefer to mix with other nonpolar substances, you know. So, knowing NF3 is polar helps us predict how it might interact with other chemicals, and that's pretty useful.
It also affects things like a substance's boiling point. Polar molecules tend to stick together more strongly due to those positive and negative attractions, which means it takes more energy to separate them and turn them into a gas. So, a polar molecule might have a higher boiling point than a nonpolar one of similar size, as a matter of fact.
Frequently Asked Questions About Polarity
People often have some common questions when they are first learning about molecular polarity, and that's perfectly normal. Here are a few that come up quite often, you know, to help clarify things further.
What makes a molecule polar?
A molecule becomes polar when it has an uneven distribution of electron density, basically. This happens if there are polar bonds present, and if the molecule's overall shape means that the electron pulls from those bonds don't cancel each other out. So, you need both aspects: polar connections and an asymmetrical arrangement, you see.
How can you tell if a molecule is polar?
To figure out if a molecule is polar, you usually look at two main things. First, check if the individual connections between the atoms are polar, by comparing their electron-pulling strengths. Second, you need to consider the molecule's three-dimensional shape. If the polar pulls add up instead of canceling, then the molecule is polar, you know.
Are all molecules with polar bonds also polar molecules?
No, not every molecule with polar bonds is a polar molecule, and this is a very important distinction to make. Take carbon dioxide, for example. It has polar bonds between carbon and oxygen, but the molecule itself is linear and symmetrical. The pulls cancel each other out, making the whole molecule nonpolar, you see. So, the shape really matters, too.
What to Explore Next in Chemistry
Understanding molecular polarity, like with NF3, is just one small piece of the amazing world of chemistry. There's so much more to discover about how atoms and molecules interact and what makes substances behave the way they do, you know.
If you're curious to learn more about how molecular shapes influence properties, you could explore concepts like VSEPR theory, which helps predict those shapes. It's a fascinating area of study, really, and it helps make sense of so much.
You can always learn more about molecular properties from reputable sources. Also, you might want to learn more about chemical bonding on our site, and you can also link to this page to find out more about us, too. There are many exciting topics waiting for you to uncover.
Solved Classify each molecule as polar or nonpolar. Polar | Chegg.com
Polar and Nonpolar Molecules
SOLVED: Are sulfur tetrafluoride (SF4) and nitrogen trifluoride (NF3