Unraveling NF3: Lewis Structure And Molecular Geometry Explained
Have you ever wondered what gives molecules their unique shapes? It's a bit like understanding why certain chess moves are made, where developing your knight early on, say with 1.Nf3, can shape the whole game. Just as a strong opening helps you control the center, a molecule's structure truly dictates its properties. You know, it's pretty important.
Today, we're going to peek into the world of nitrogen trifluoride, or NF3. This particular molecule, you know, has a really interesting setup that affects how it behaves. We'll explore its Lewis structure and then see how that drawing helps us figure out its actual shape in space, which is pretty cool.
Getting a handle on NF3's structure is pretty important for chemistry students and anyone curious about how atoms connect. It helps make sense of things like polarity and reactivity, which are, you know, big deals in chemical reactions. So, let's get started on this little exploration, shall we?
Table of Contents
- What is NF3? A Quick Look
- Drawing the NF3 Lewis Structure: A Step-by-Step Guide
- VSEPR Theory: Unlocking NF3's Molecular Geometry
- Why NF3's Shape Matters: Properties and Uses
- NF3 Compared to NH3: A Shape Story
- Common Questions About NF3's Structure
- Conclusion: The Shape of Things
What is NF3? A Quick Look
NF3 is the chemical formula for nitrogen trifluoride. It's a compound made of one nitrogen atom and three fluorine atoms. This substance is a colorless, non-flammable gas. It has a rather distinct odor, too, which is interesting. People use it in making electronics, particularly in cleaning equipment for microchips, so it has real-world uses.
Knowing about NF3's structure helps us predict how it will react with other chemicals. Just like understanding the "London System" in chess, which is a very solid opening because white can use the same basic setup for almost any situation, understanding a molecule's basic structure provides a solid foundation for predicting its behavior. It's a fundamental piece of knowledge, actually.
Drawing the NF3 Lewis Structure: A Step-by-Step Guide
Drawing a Lewis structure is like creating a blueprint for a molecule. It shows how atoms connect and where electrons sit. This is a very important first step to figuring out a molecule's shape, you know, its geometry. Let's walk through the steps for NF3, which is a good example.
Counting All the Valence Electrons
First, we need to count the total number of valence electrons. These are the electrons in the outermost shell of an atom, the ones that get involved in bonding. Nitrogen is in Group 15 of the periodic table, so it has 5 valence electrons. Fluorine is in Group 17, meaning it has 7 valence electrons. Since we have one nitrogen and three fluorines, we calculate the total like this: (1 Nitrogen atom * 5 electrons/atom) + (3 Fluorine atoms * 7 electrons/atom). That works out to 5 + 21, giving us a grand total of 26 valence electrons. This number is really important, you know, for everything that comes next.
Finding the Central Atom
Next, we pick the central atom. The central atom is usually the least electronegative atom, except for hydrogen. Nitrogen is less electronegative than fluorine. So, nitrogen will sit in the middle, and the three fluorine atoms will surround it. This is a pretty common rule in chemistry, you know, when you're setting up these structures.
Drawing Single Bonds
Now, we draw single bonds between the central nitrogen atom and each of the three fluorine atoms. Each single bond uses two electrons. Since we have three single bonds, we've used 3 * 2 = 6 electrons so far. We started with 26 total valence electrons, and we've used 6, so we have 20 electrons left to place. This is a crucial step, you know, to make sure you're accounting for all the electrons.
Adding Lone Pairs to Outer Atoms
After placing the single bonds, we distribute the remaining electrons as lone pairs on the outer atoms first. Each fluorine atom needs to have an octet, meaning eight electrons around it. Since each fluorine already has 2 electrons from its single bond with nitrogen, it needs 6 more electrons to complete its octet. So, we place three lone pairs (6 electrons) on each of the three fluorine atoms. That's 3 fluorine atoms * 6 electrons/fluorine = 18 electrons used. We had 20 electrons left, and now we've used 18 of them. This leaves us with 2 electrons still to place, which is just a little bit left.
Placing Remaining Lone Pairs on the Central Atom
The last step for placing electrons is to put any remaining electrons on the central atom. We have 2 electrons left over. The central nitrogen atom currently has 6 electrons around it (2 from each of its three single bonds). It needs 8 electrons for a full octet. Placing the remaining 2 electrons on the nitrogen atom as one lone pair completes its octet. So, the nitrogen now has 6 electrons from bonds plus 2 from the lone pair, making 8 electrons total. This is pretty much the final electron placement.
Checking for Full Octets
Finally, we check to make sure all atoms have a full octet (8 electrons), except for hydrogen, which only needs 2. Each fluorine atom has 6 electrons from its three lone pairs and 2 electrons from its single bond, totaling 8. The nitrogen atom has 2 electrons from each of its three single bonds (6 total) and 2 electrons from its lone pair, also totaling 8. So, everyone is happy with a full octet. This confirms our Lewis structure is correct, you know, for the electron count.
VSEPR Theory: Unlocking NF3's Molecular Geometry
The Lewis structure shows us the connections, but it doesn't tell us the actual 3D shape. For that, we use something called VSEPR theory. VSEPR stands for Valence Shell Electron Pair Repulsion. It's a simple idea: electron groups around a central atom try to get as far away from each other as possible to minimize repulsion. This pushing away, you know, creates the molecule's shape. It's kind of like how chess pieces occupy squares, trying to control space and avoid being too close to enemy pieces.
Electron Domain Geometry: The Overall Arrangement
First, we look at the electron domain geometry. An electron domain is any region where electrons are concentrated. This includes single bonds, double bonds, triple bonds, and lone pairs. For NF3, the central nitrogen atom has three single bonds to fluorine atoms and one lone pair of electrons. So, the nitrogen has a total of four electron domains around it (three bonding domains and one lone pair domain). With four electron domains, the electron domain geometry is tetrahedral. This means these four regions of electron density will point towards the corners of a tetrahedron, which is a shape with four faces, you know, a pyramid-like structure.
Molecular Geometry: The Actual Shape
Now, for the molecular geometry, we only consider the positions of the atoms, not the lone pairs. Even though lone pairs take up space and influence the overall electron domain geometry, they aren't "atoms" themselves. In NF3, we have a central nitrogen atom and three fluorine atoms bonded to it, plus one lone pair on the nitrogen. Because of that lone pair pushing the bonding pairs away, the actual shape of the atoms is not tetrahedral. Instead, it's a trigonal pyramidal shape. This means the nitrogen atom is at the top of a pyramid, and the three fluorine atoms form the base. It's a bit like a small tripod, you know, with a little extra bulk on top.
Understanding NF3's Bond Angles
In a perfect tetrahedral arrangement, the bond angles would be 109.5 degrees. However, lone pairs take up more space than bonding pairs because they are only attracted to one nucleus, not two. This extra repulsion from the lone pair on the nitrogen atom pushes the N-F bonding pairs closer together. As a result, the F-N-F bond angles in NF3 are slightly less than 109.5 degrees, typically around 102.5 degrees. This is a very common effect, you know, when lone pairs are present. It's a subtle but important detail.
Why NF3's Shape Matters: Properties and Uses
The specific trigonal pyramidal shape of NF3 has big implications for its properties. This shape, combined with the difference in electronegativity between nitrogen and fluorine, makes NF3 a polar molecule. Polarity means there's an uneven distribution of electron density, creating a slight positive end and a slight negative end. This is a bit like how the "Ruy Lopez" in chess is rich in strategy; the molecular geometry of NF3 is rich in its implications for its chemical behavior. This polarity influences how NF3 interacts with other molecules, including its solubility and boiling point. It's a rather significant feature.
NF3 is used in the semiconductor industry. Its unique properties, which come from its structure, make it good for plasma etching and cleaning. It helps make the tiny, intricate circuits on computer chips. Understanding its molecular geometry helps scientists and engineers predict its behavior in these industrial processes, ensuring things work as they should. It's actually a pretty cool application of basic chemistry.
The stability of NF3 also relates to its structure. The strong N-F bonds contribute to its overall stability. This stability is important for its use in manufacturing environments, where it needs to be reliable. Just like developing the king's knight in chess helps remove the risk of early queen checks, understanding the stable structure of NF3 removes risks in its industrial application. It's a very practical aspect.
Knowing about the molecular geometry of NF3 also helps us understand its environmental impact. NF3 is a potent greenhouse gas, meaning it traps heat in the atmosphere. Its stability, a direct result of its structure, means it stays in the atmosphere for a long time. So, its shape and properties have consequences far beyond the lab. This is something we, you know, need to be aware of.
NF3 Compared to NH3: A Shape Story
It's often helpful to compare NF3 with ammonia (NH3) to truly grasp the nuances of molecular geometry. Both molecules have a central nitrogen atom with one lone pair and three atoms bonded to it. So, both have a tetrahedral electron domain geometry and a trigonal pyramidal molecular geometry. This is a good starting point for comparison.
However, there's a key difference in their polarity. NH3 is very polar, with a large dipole moment. This is because nitrogen is more electronegative than hydrogen, pulling electron density towards itself and the lone pair also contributes strongly. In NF3, fluorine is much more electronegative than nitrogen. The individual N-F bond dipoles point towards the fluorine atoms. While there's a lone pair, the strong pull of the fluorines tends to counteract the lone pair's contribution to the overall molecular dipole moment, making NF3 less polar than NH3, even though it's still polar. It's a subtle but important distinction, you know, in how the electron pulls balance out.
This difference in polarity, which stems from the specific atoms and their arrangement, affects their boiling points, solubility, and how they interact with other substances. NH3, for instance, forms strong hydrogen bonds, which NF3 does not. This is a direct consequence of their specific structures and electron distributions. It's quite interesting, you know, how these small differences lead to big changes.
So, while both NF3 and NH3 share the same basic trigonal pyramidal shape, the specific atoms involved and their electronegativity differences mean their overall properties are quite different. This highlights how important it is to consider all aspects of a molecule's makeup, not just its general geometry. Learn more about on our site, and link to this page .
Common Questions About NF3's Structure
People often have questions about NF3's structure, especially when they are first learning about it. Here are a few common ones, you know, that come up pretty often.
What is the hybridization of the central atom in NF3?
The central nitrogen atom in NF3 has four electron domains: three bonding pairs and one lone pair. When an atom has four electron domains, its hybridization is sp3. This means one s orbital and three p orbitals on the nitrogen atom mix to form four new, equivalent sp3 hybrid orbitals. These hybrid orbitals then point towards the corners of a tetrahedron, which is pretty much what we saw with the electron domain geometry.
Is NF3 a polar or nonpolar molecule?
NF3 is a polar molecule. While the individual N-F bonds are polar (fluorine pulls electrons away from nitrogen), and the molecule has a trigonal pyramidal shape, the overall molecule has a net dipole moment. The lone pair on nitrogen contributes to this polarity, and the bond dipoles do not completely cancel each other out due to the molecule's specific shape. This means one side of the molecule is slightly negative, and the other is slightly positive. It's a very clear example of how shape affects polarity.
How many lone pairs are on the central atom of NF3?
There is one lone pair of electrons on the central nitrogen atom in NF3. This lone pair is crucial for determining the molecule's trigonal pyramidal shape, as it pushes the bonding pairs away, reducing the bond angles from a perfect tetrahedral angle. This single lone pair, you know, makes a big difference in the final shape.
Conclusion: The Shape of Things
Understanding the NF3 Lewis structure and its molecular geometry is a fundamental step in chemistry. We've seen how counting valence electrons, identifying the central atom, and systematically placing bonds and lone pairs leads us to the correct Lewis structure. Then, applying VSEPR theory helps us predict the molecule's actual 3D shape, which for NF3 is trigonal pyramidal with bond angles around 102.5 degrees. This shape, in turn, influences its polarity and its behavior in various applications, like making microchips. It's a rather neat connection.
The systematic approach to understanding molecular structures, you know, is a bit like mastering the "Italian Game" in chess, which starts with specific moves like 1.e4 e5 2.Nf3 Nc6 3.Bc4. Each step builds on the last, creating a complete picture. This knowledge helps us predict how chemicals will act and even design new materials. For more details on molecular shapes, you can always consult a reputable chemistry resource like this one.
Keep exploring the shapes of molecules around you. Every molecule has a story told by its structure, and learning to read that story opens up a whole new way of looking at the world. It's pretty amazing, actually, what you can learn just by looking at how atoms connect.
Today is October 26, 2023, and the principles of Lewis structures and VSEPR theory remain foundational to understanding chemical compounds. These concepts are always relevant, you know, for anyone studying chemistry.
So, the next time you hear about a chemical, think about its shape. It tells you so much about what it does. It's a very simple idea with huge implications.
[Solved] Lab # 10: Molecular Geometry Lab Lewis Structure Molecular
Lab 9 - Bonding and Molecular Shapes Compound Lewis Structure Report
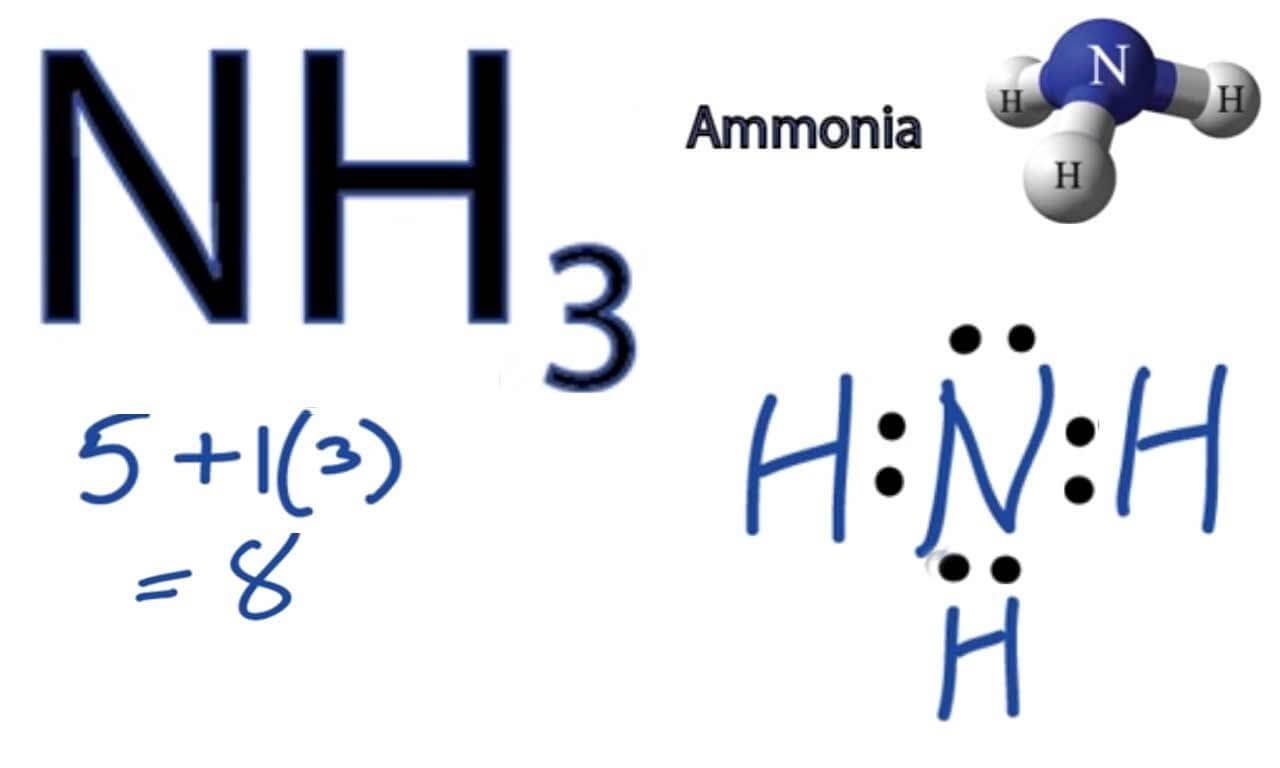
N3 Lewis Structure Molecular Geometry Hybridization B - vrogue.co