Unraveling The NF3 Molecular Structure: A Look At Nitrogen Trifluoride
Have you ever looked at a chemical formula like NF3 and wondered what it actually means? It's almost like seeing a secret code, isn't it? Well, in the fascinating world of chemistry, these simple combinations of letters and numbers tell us so much about how atoms come together to form unique substances. Understanding the basic building blocks of matter, like the way nitrogen and fluorine link up in NF3, helps us make sense of the materials all around us. It's a bit like learning the rules of a game; once you know them, you can start to see how everything fits.
This particular compound, NF3, is called nitrogen trifluoride. It's a pretty interesting molecule, and its structure has some really important implications for how it behaves. You see, the way atoms arrange themselves isn't random; there are specific forces and rules that guide their connections. This arrangement, which we call molecular structure, really dictates a compound's properties, like whether it's a gas or a liquid, how it reacts, and even what it's used for in our daily lives. So, knowing about the nf3 molecular structure is a key piece of information.
Today, on June 10, 2024, we're going to take a closer look at NF3, breaking down its shape and how its atoms interact. We'll explore why it forms the way it does and what makes it special. It's a rather neat example of how fundamental chemical principles play out in a real-world molecule, and you know, it's pretty cool to think about how tiny particles behave in such predictable ways.
Table of Contents
- What is Nitrogen Trifluoride (NF3)?
- Drawing the Lewis Structure of NF3
- Understanding the Molecular Shape: VSEPR Theory and NF3
- The Bonding Story: Hybridization in NF3
- NF3's Polarity and Its Impact on Properties
- Real-World Uses and Environmental Considerations of NF3
- Frequently Asked Questions About NF3 Molecular Structure
- Putting It All Together
What is Nitrogen Trifluoride (NF3)?
So, what exactly is NF3? Well, it's a covalent compound, which means its atoms share electrons to form bonds. This is different from ionic compounds, where electrons are transferred. Our source material tells us it's a covalent compound consisting of one nitrogen atom bonded to three fluorine atoms. This is a very important detail, as it sets the stage for everything else we'll discover about its shape and behavior. Nitrogen, you see, is a nonmetal, and fluorine is also a nonmetal, so their natural inclination is to share electrons rather than give them up or take them completely. This electron sharing creates a strong link between them.
The "tri" in trifluoride just means there are three fluorine atoms connected to that one nitrogen atom. It's a simple name that gives us a lot of information right away. These atoms come together in a very specific way, which we will explore in detail. Understanding this basic composition is the first step to truly appreciating the nf3 molecular structure. It's a bit like knowing the ingredients before you start baking; you need to know what you're working with, right?
Nitrogen, which is element number seven on the periodic table, usually likes to form three bonds, and it often has a pair of unshared electrons, what we call a lone pair. Fluorine, element number nine, is very good at attracting electrons and typically forms just one bond. These tendencies are key to how the NF3 molecule arranges itself. This fundamental understanding of each atom's preference really helps to explain why the compound forms as it does, and that, too, is a big part of its overall structure.
Drawing the Lewis Structure of NF3
To really see the nf3 molecular structure, we often start by drawing something called a Lewis structure. This drawing helps us visualize where the electrons are located within the molecule, showing both the shared electrons in bonds and any unshared electron pairs, also known as lone pairs. It's a helpful tool for chemists and anyone trying to figure out how atoms are connected. You might think of it as a blueprint for the molecule, showing us the fundamental connections.
First, we count the total number of valence electrons. Nitrogen has five valence electrons, and each of the three fluorine atoms has seven valence electrons. So, that's 5 + (3 * 7) = 5 + 21 = 26 total valence electrons. Then, we pick a central atom. Nitrogen is less electronegative than fluorine, so it typically sits in the middle. We connect the central nitrogen atom to the three fluorine atoms with single bonds. This uses up 2 electrons per bond, so 3 * 2 = 6 electrons are used for bonding.
After placing the bonding electrons, we distribute the remaining electrons to satisfy the octet rule for each atom, meaning each atom wants eight electrons around it. We have 26 - 6 = 20 electrons left. Each fluorine atom needs 6 more electrons to complete its octet (it already has 2 from the bond). So, 3 fluorine atoms * 6 electrons/fluorine = 18 electrons. We place these 18 electrons as lone pairs on the fluorine atoms. Each fluorine atom now has 8 electrons around it: 2 from the bond and 6 from its three lone pairs. That is quite a neat arrangement, isn't it?
Finally, we check the central nitrogen atom. It has 6 electrons from the three single bonds. It needs 2 more electrons to complete its octet. We have 20 - 18 = 2 electrons remaining. These last 2 electrons go on the nitrogen atom as a lone pair. So, the Lewis structure shows a central nitrogen atom bonded to three fluorine atoms, with one lone pair on the nitrogen and three lone pairs on each fluorine atom. This detailed view is very important for understanding the next step, which is figuring out the actual three-dimensional shape of the molecule. This whole process, you know, gives us a very clear picture of the electron arrangement.
Understanding the Molecular Shape: VSEPR Theory and NF3
Now that we have the Lewis structure, we can figure out the actual three-dimensional shape of the nf3 molecular structure. For this, we use something called VSEPR theory, which stands for Valence Shell Electron Pair Repulsion theory. This theory is actually quite intuitive: it says that electron pairs, whether they are in bonds or are lone pairs, repel each other. They try to get as far away from each other as possible to minimize these repulsions. This repulsion, basically, pushes the atoms into a specific geometry.
In NF3, the central nitrogen atom has four "electron domains" around it. These domains are the three single bonds to the fluorine atoms and the one lone pair of electrons on the nitrogen. So, we have three bonding pairs and one lone pair. According to VSEPR theory, four electron domains will arrange themselves in a tetrahedral electron geometry to be as far apart as possible. However, the molecular shape, which is what we see when we look at the atoms themselves, is different because the lone pair takes up space but isn't an atom.
The lone pair of electrons on the nitrogen atom exerts a bit more repulsion than the bonding pairs. This increased repulsion pushes the three bonding fluorine atoms closer together. As a result, the nf3 molecular structure takes on a trigonal pyramidal shape. Imagine a pyramid with the nitrogen atom at the top (or apex) and the three fluorine atoms forming the base. The lone pair of electrons sits above the nitrogen, pushing the bonds downwards. This is very much like a tripod, but with one leg missing, and where that missing leg would be, there's a kind of invisible "push" from the lone pair. That, you know, really impacts the overall look.
This trigonal pyramidal shape is a classic example of how lone pairs influence molecular geometry. If there were no lone pair on the nitrogen, and instead four bonding atoms, it would be a perfect tetrahedron. But the presence of that one lone pair distorts the shape, making it pyramidal. This is a crucial aspect of the nf3 molecular structure and explains many of its physical and chemical properties. It's a rather neat illustration of how subtle electron interactions can lead to distinct three-dimensional arrangements, and that, in a way, is pretty cool to observe.
The Bonding Story: Hybridization in NF3
To get an even deeper look at the nf3 molecular structure, we can talk about something called hybridization. This concept helps us explain how atomic orbitals, which are the regions where electrons are likely to be found, mix and rearrange themselves to form new, equivalent hybrid orbitals that are better suited for bonding. It's a bit like combining different ingredients to make a new, more effective mixture. For the nitrogen atom in NF3, we consider its electron configuration.
Nitrogen's electron configuration is 1s² 2s² 2p³. In its valence shell, it has one 2s orbital and three 2p orbitals. When nitrogen forms bonds in NF3, one 2s orbital and all three 2p orbitals combine to form four new, identical hybrid orbitals. We call this sp³ hybridization. These four sp³ hybrid orbitals point towards the corners of a tetrahedron, which, you know, aligns perfectly with the electron domain geometry we talked about earlier. One of these sp³ hybrid orbitals will hold the lone pair of electrons, and the other three will form single bonds with the fluorine atoms.
Each of the three fluorine atoms, on the other hand, uses one of its p orbitals to overlap with an sp³ hybrid orbital from the nitrogen atom. This overlap creates the single covalent bonds between nitrogen and fluorine. These bonds are sigma bonds, which are the strongest type of covalent bond formed by direct overlap of orbitals. The electrons in these sigma bonds are located directly between the nuclei of the bonded atoms. This is a very stable way for them to connect, and it's quite typical for single bonds.
The bond angles in NF3 are actually slightly less than the ideal 109.5 degrees for a perfect tetrahedron, typically around 102.5 degrees. This reduction in bond angle is due to the greater repulsion exerted by the lone pair of electrons on the nitrogen atom compared to the bonding pairs. The lone pair takes up more space and pushes the bonding pairs closer together. So, the hybridization concept really supports our understanding of the trigonal pyramidal shape and the specific angles within the nf3 molecular structure. It gives us a very detailed picture of how electrons are shared and arranged.
NF3's Polarity and Its Impact on Properties
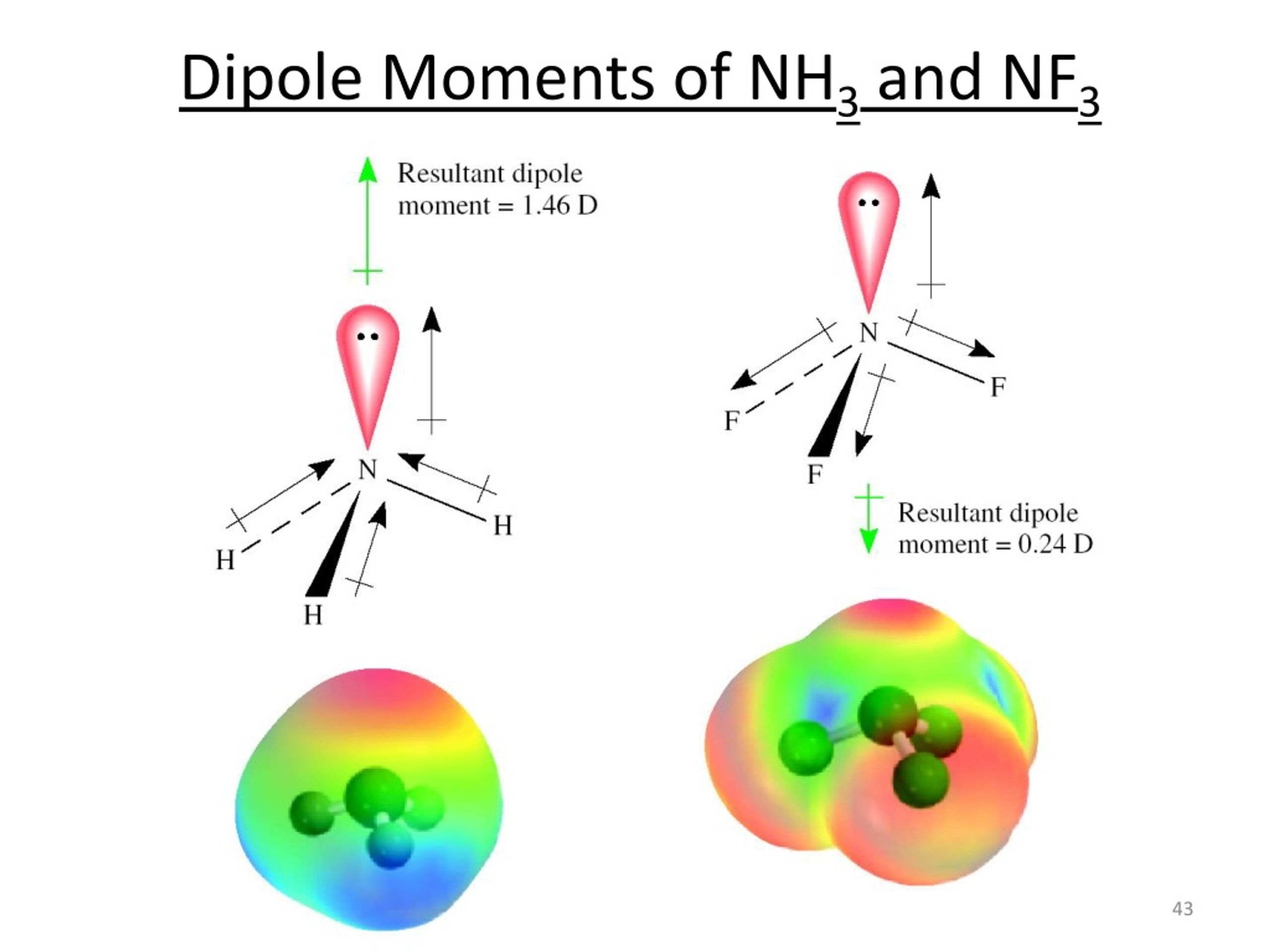
The nf3 molecular structure also tells us a lot about its polarity. A molecule is considered polar if it has a net dipole moment, meaning there's an uneven distribution of electron density across the molecule, creating a slightly positive end and a slightly negative end. This happens when there are polar bonds and the molecular shape is asymmetrical, preventing the individual bond dipoles from canceling each other out. It's like having a tug-of-war where one side is clearly stronger, pulling the rope in its direction.
First, let's look at the bonds themselves. Fluorine is much more electronegative than nitrogen. Electronegativity is an atom's ability to attract shared electrons in a covalent bond. Because fluorine is so good at pulling electrons, the electrons in each N-F bond are pulled closer to the fluorine atom. This creates a partial negative charge on each fluorine atom and a partial positive charge on the nitrogen atom. So, each N-F bond is indeed a polar bond. This is a very important point, as it's the starting place for understanding the molecule's overall polarity.
Now, we consider the overall molecular shape. Since NF3 has a trigonal pyramidal shape, it's asymmetrical. The lone pair on the nitrogen atom points in one direction, and the three N-F bonds point downwards. The individual bond dipoles from the N-F bonds do not cancel each other out because of this asymmetry. Instead, they add up to create a net dipole moment for the entire molecule. The nitrogen atom, with its lone pair, acts like the negative pole, and the fluorine atoms, while individually negative, contribute to an overall dipole that doesn't cancel out due to the geometry. That, you know, is pretty critical.
Because NF3 is a polar molecule, it has certain properties. Polar molecules tend to have stronger intermolecular forces than nonpolar molecules of similar size. This can affect things like boiling points and solubility. NF3 is a colorless, odorless gas at room temperature. It's also known for being a very stable compound, which means it doesn't easily react with other substances. This stability, coupled with its gaseous nature, makes it quite useful in certain industrial applications, which we'll touch on next. The polarity, in a way, helps explain why it behaves as it does in different environments.
Real-World Uses and Environmental Considerations of NF3
The unique nf3 molecular structure and its properties make it valuable in several industrial applications. One of its main uses is in the semiconductor industry. It acts as an etching agent, which means it's used to precisely remove thin layers of material during the manufacturing of microchips and other electronic components. Its stability and gaseous form make it ideal for this kind of precise work, where purity and control are very important. It's a rather critical component in making the tiny, complex circuits that power our modern devices, so it's more common than you might think.
Beyond semiconductors, NF3 is also used as a cleaning agent. For example, it helps clean deposition chambers in the production of flat panel displays, like those found in LCD screens, and also in the manufacturing of solar panels. In these processes, thin films are deposited, and NF3 helps remove any unwanted residues or byproducts that build up in the equipment. This keeps the manufacturing process efficient and ensures the quality of the final products. It's a very specific job, and NF3 does it quite well.
However, while NF3 is useful, it also has a significant environmental consideration. It is a very powerful greenhouse gas. This means it traps heat in the Earth's atmosphere much more effectively than carbon dioxide, contributing to global warming. Its global warming potential (GWP) is thousands of times higher than that of CO2 over a 100-year period. So, while it's not as abundant as CO2, its impact per molecule is quite large. This is something that, you know, scientists and policymakers are quite concerned about.
Because of its environmental impact, there's a lot of effort to reduce NF3 emissions from industrial processes. Companies are working on improving manufacturing techniques to capture and destroy NF3 before it's released into the atmosphere, or to find alternative compounds that are less harmful. So, while the nf3 molecular structure gives it useful properties, its environmental footprint means its use needs careful management. It's a good example of how chemistry, industry, and environmental science are all interconnected, and it's something we really need to keep an eye on for the future. You can learn more about chemical compounds and their properties from reputable chemistry organizations.
Frequently Asked Questions About NF3 Molecular Structure
What is the shape of the NF3 molecule?
The NF3 molecule has a trigonal pyramidal shape. This means the nitrogen atom sits at the top of a pyramid, and the three fluorine atoms form the base. This shape is due to the presence of one lone pair of electrons on the nitrogen atom, which pushes the bonding pairs downwards. It's a rather distinct arrangement, you know, that really defines its overall form.
Is NF3 a polar or nonpolar molecule?
NF3 is a polar molecule. Each N-F bond is polar because fluorine is more electronegative than nitrogen, pulling electrons towards itself. Because the molecule has a trigonal pyramidal shape, which is asymmetrical, these individual bond polarities do not cancel out. Instead, they add up, creating a net dipole moment for the entire molecule. This asymmetry, you know, is pretty important for its polarity.
How many lone pairs are on the central nitrogen atom in NF3?
There is one lone pair of electrons on the central nitrogen atom in NF3. This lone pair is crucial for determining the molecule's trigonal pyramidal shape, as it exerts a stronger repulsion on the bonding pairs than the bonding pairs do on each other. This lone pair, you know, is a key feature of its structure.
Putting It All Together
We've taken a pretty detailed look at the nf3 molecular structure, from its basic composition to its intricate three-dimensional shape and how that influences its behavior. We saw how one nitrogen atom and three fluorine atoms come together, sharing electrons in a covalent bond. The Lewis structure helps us map out these electron arrangements, showing us where the bonds are and where the unshared electron pairs, the lone pairs, reside. It's a very foundational piece of information, and it really sets the stage for everything else.
Then, using VSEPR theory, we discovered that the molecule isn't flat but forms a trigonal pyramid. This specific shape is a direct result of the lone pair on the nitrogen atom pushing the bonding atoms into that particular configuration. We also touched on hybridization, which explains how the atomic orbitals rearrange themselves to make these bonds. All these pieces, you know, fit together to give us a complete picture of why NF3 looks and acts the way it does. Understanding these concepts helps us appreciate the complexity of even seemingly simple chemical compounds, and you can learn more about chemical bonding on our site, or explore other molecular structures right here.
Lab 9 - Bonding and Molecular Shapes Compound Lewis Structure Report
PPT - Five Basic Molecular Structures PowerPoint Presentation, free
Nf3 Molecular Geometry Bond Angles