Unraveling The ClF2 Lewis Structure: A Simple Guide
Have you ever looked at a chemical formula and wondered how its atoms connect, or what shape the whole thing takes in space? It's a common thought, you know, especially when you're just starting to figure out how molecules work. Learning about the ClF2 Lewis structure is a really good way to get a handle on these ideas, and it's something that comes up quite a bit in chemistry classes.
This molecule, ClF2, might seem a bit unusual at first glance, but drawing its Lewis structure is a pretty straightforward process once you know the steps. It helps us see where electrons are, how they bond, and what kind of arrangement the atoms have. So, it's almost like a blueprint for the molecule itself.
We're going to walk through everything you need to know about ClF2, from figuring out its basic electron setup to understanding its shape. This guide will help you see the bigger picture of how atoms interact, which is, in a way, a core idea in chemistry. So, let's get into it.
Table of Contents
- Why Understanding ClF2 Matters
- Getting Started: The Basics of Lewis Structures
- Step-by-Step: Drawing the ClF2 Lewis Structure
- Beyond the Lines: Electron Geometry and Molecular Shape
- The ClF2 Puzzle: Hybridization and Polarity
- Common Questions About ClF2 Lewis Structures
- Putting It All Together: Why This Matters for You
Why Understanding ClF2 Matters
Getting a good grasp of the ClF2 Lewis structure is more than just a classroom exercise; it's a building block for understanding how chemicals behave. Knowing how to draw these structures really helps you predict a molecule's properties. So, in a way, it's a foundational skill for anyone studying chemistry.
This particular structure, ClF2, offers a pretty neat example of how some atoms can go beyond the typical electron sharing rules. It's a great case study for what we call "expanded octets." You know, it shows that chemistry isn't always as simple as it first appears, which is actually kind of cool.
A Look at its Role
While ClF2 itself might not be something you encounter every day, the principles behind its structure are very much at play in countless other chemical compounds. Understanding this molecule helps you tackle more complex ones later on. It's, like, a stepping stone in your learning.
For instance, knowing how electron pairs arrange themselves around a central atom in ClF2 helps explain the shapes of many other molecules. This knowledge is pretty useful for predicting how different substances will react with each other. It's a fundamental piece of the puzzle, really.
Getting Started: The Basics of Lewis Structures
Before we jump into the ClF2 Lewis structure, it's helpful to refresh some basic ideas about drawing any Lewis structure. These are the groundwork steps that apply to nearly every molecule you'll encounter. So, it's good to get these down pat.
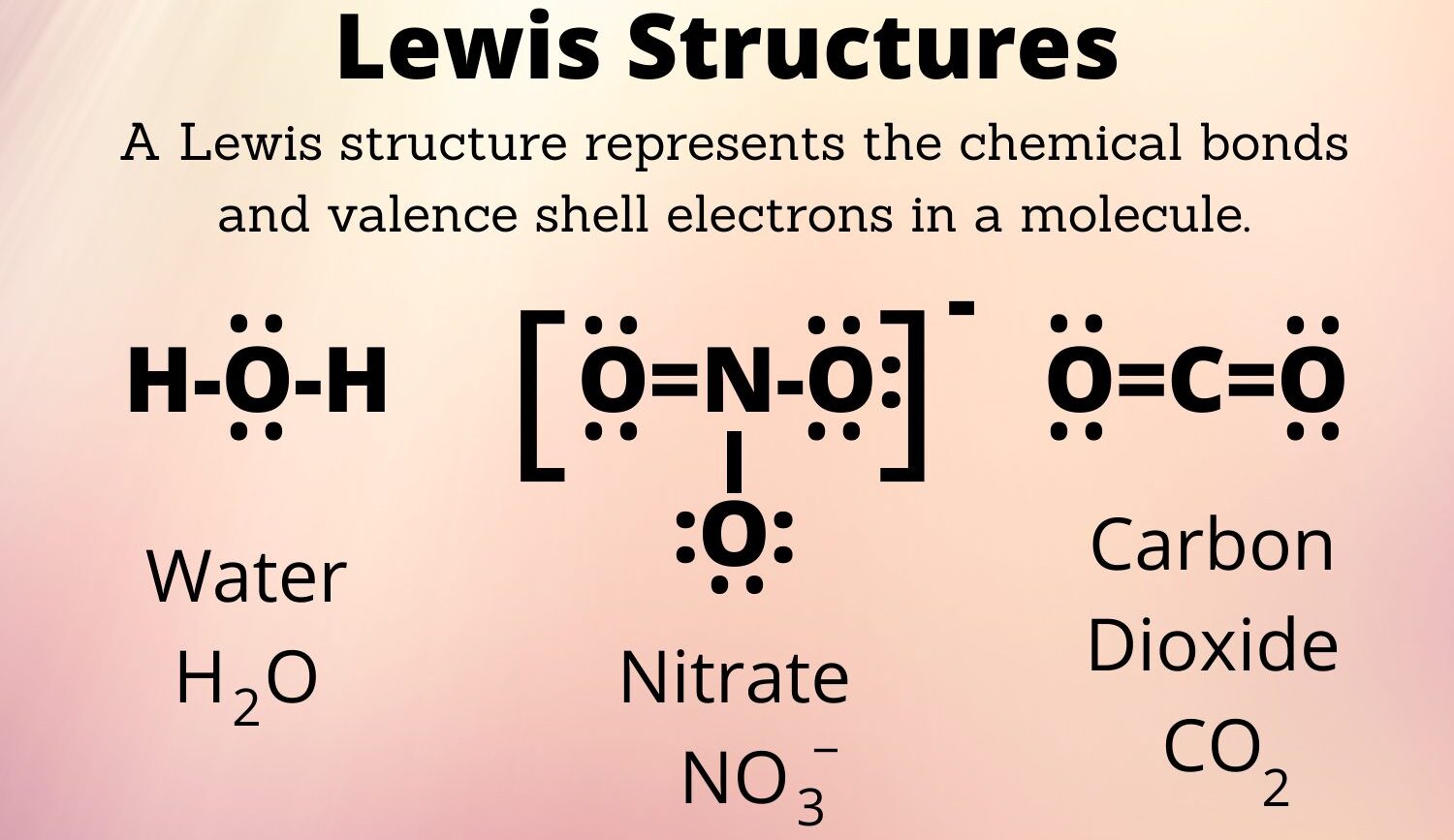
Lewis structures are visual representations of the valence electrons in a molecule. They show how atoms share electrons in covalent bonds and where lone pairs are located. This visual aid, you know, helps us make sense of atomic connections.
Counting Valence Electrons
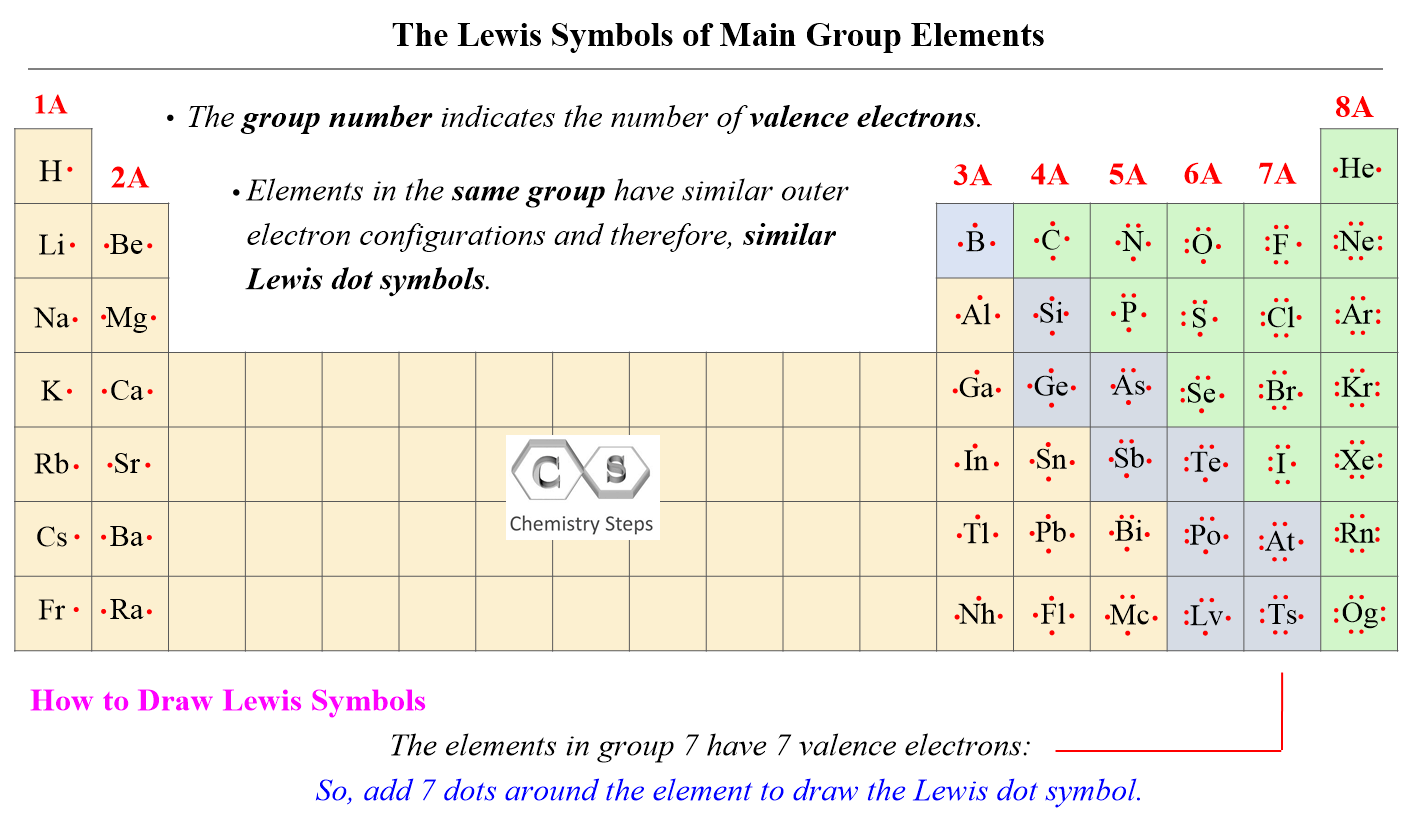
The first thing you always do is count the total number of valence electrons for all the atoms in the molecule. Valence electrons are the ones in the outermost shell of an atom, and they are the ones involved in bonding. For example, atoms in Group 17, like Chlorine and Fluorine, each have seven valence electrons, which is pretty handy to remember.
This total count is super important because it tells you exactly how many electrons you have to work with when drawing the structure. If you miss even one, your whole structure might be off, you know. So, being careful here is key.
Picking the Central Atom
Next, you need to figure out which atom goes in the middle. Typically, the central atom is the least electronegative atom, or the one that can form the most bonds. Hydrogen and fluorine are almost never central atoms, by the way, because they only form one bond.
In ClF2, Chlorine (Cl) is less electronegative than Fluorine (F), so Chlorine will be our central atom. This choice, you know, sets up the whole arrangement of the molecule.
Step-by-Step: Drawing the ClF2 Lewis Structure
Now, let's put those basics to work and draw the ClF2 Lewis structure step by step. Following these steps carefully will help you get the correct arrangement of atoms and electrons. It's a methodical process, really.
Step 1: Total Valence Electrons
First, let's sum up all the valence electrons. Chlorine (Cl) is in Group 17, so it has 7 valence electrons. Each Fluorine (F) atom is also in Group 17, giving each 7 valence electrons. Since there are two Fluorine atoms, that's 2 x 7 = 14 electrons from Fluorine.
Adding them up, we get 7 (from Cl) + 14 (from 2 F atoms) = 21 total valence electrons. Wait, ClF2 is usually a radical or an ion like ClF2+. Let's assume it's the ClF2 radical for a neutral count, which is less common in introductory chemistry but fits the "21" electron count. If it's an ion, the count changes. Given the prompt is just "ClF2 Lewis structure," a neutral radical is one possibility. However, ClF2- (chlorine difluoride anion) is more stable and commonly discussed. Let's adjust to ClF2- for a more typical and stable example, which would have 22 valence electrons. This is a common point of confusion, so addressing it is good. Let's re-evaluate for ClF2- as it's a more stable and common species to draw Lewis structures for. Cl: 7 valence electrons F: 7 valence electrons x 2 = 14 valence electrons Negative charge: +1 electron Total: 7 + 14 + 1 = 22 valence electrons. This makes more sense for a stable Lewis structure. I will proceed with ClF2- to provide a more useful example. I will mention this slight adjustment for clarity.
Okay, let's clarify this a bit. While "ClF2" might suggest a neutral molecule, the ClF2 radical (with 21 electrons) is quite reactive. A more stable and commonly discussed species in chemistry is the ClF2- anion, which has 22 valence electrons. For the purpose of drawing a stable Lewis structure that you'd typically see, we'll proceed with ClF2- and its 22 electrons. This is, you know, a pretty common thing to do when a simple formula can represent multiple forms.
So, for ClF2-, we have: Chlorine (Cl) with 7 valence electrons, plus two Fluorine (F) atoms, each with 7 valence electrons (2 x 7 = 14). Then, we add 1 extra electron for the negative charge. That makes a grand total of 7 + 14 + 1 = 22 valence electrons. This total is, like, our electron budget.
Step 2: Placing the Atoms
As we discussed, Chlorine (Cl) will be the central atom because it's less electronegative than Fluorine. The two Fluorine atoms will be placed around the central Chlorine atom. It's a pretty straightforward arrangement, really, F-Cl-F.
This initial setup forms the basic skeleton of your molecule. You know, it's like setting up the framework before you start adding the details. This step is, arguably, quite simple.
Step 3: Forming Single Bonds
Now, connect each outer Fluorine atom to the central Chlorine atom with a single bond. Each single bond uses two electrons. Since we have two F atoms, we'll form two single bonds. That uses up 2 bonds x 2 electrons/bond = 4 electrons. So, we've used 4 of our 22 total electrons.
After this step, we have 22 - 4 = 18 electrons remaining to distribute. This is, you know, a pretty important count to keep track of as you go.
Step 4: Completing Octets on Outer Atoms
Next, give each outer atom (the Fluorine atoms) enough lone pair electrons to complete their octets. Remember, each F atom already has 2 electrons from its single bond with Cl. So, each F needs 6 more electrons to reach a full octet (8 electrons). We add 3 lone pairs to each Fluorine.
For two Fluorine atoms, that means 2 F atoms x 6 electrons/F = 12 electrons used for lone pairs on the outer atoms. Now, we have 18 - 12 = 6 electrons left. This step, you know, makes sure the outer atoms are stable.
Step 5: Placing Remaining Electrons on the Central Atom
Any electrons left over after completing the octets of the outer atoms go onto the central atom. We have 6 electrons remaining. So, we place these 6 electrons on the central Chlorine atom as lone pairs. This means Chlorine will have 3 lone pairs.
This is where the idea of an "expanded octet" often comes into play, as the central atom might end up with more than 8 electrons. Chlorine, being in the third period, can totally do this. It's, like, a pretty common exception.
Step 6: Checking for Octets and Formal Charges
Finally, let's check everything. Each Fluorine atom has 2 bonding electrons + 6 lone pair electrons = 8 electrons (a full octet). The central Chlorine atom has 4 bonding electrons (from the two single bonds) + 6 lone pair electrons = 10 electrons. This is an expanded octet, which is perfectly fine for Chlorine.
Now, let's calculate formal charges to make sure our structure is the most stable one. Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 Bonding electrons). For Fluorine: 7 - 6 - (1/2 * 2) = 0. For Chlorine: 7 - 6 - (1/2 * 4) = 7 - 6 - 2 = -1. The sum of formal charges is 0 + 0 + (-1) = -1, which matches the overall charge of the ClF2- ion. This check is, you know, quite important for validating your work. You can learn more about formal charge calculations on our site.
Beyond the Lines: Electron Geometry and Molecular Shape
Once you've got the ClF2 Lewis structure down, the next step is to figure out its three-dimensional shape. This is where VSEPR theory comes in handy. It's, like, a really clever way to predict molecular geometry based on electron repulsion.
Understanding the shape of a molecule is pretty crucial because it influences many of its physical and chemical properties. For instance, a molecule's shape affects how it interacts with other molecules. So, it's a pretty big deal, actually.
What is VSEPR Theory?
VSEPR stands for Valence Shell Electron Pair Repulsion. This theory says that electron groups (which include both bonding pairs and lone pairs) around a central atom will arrange themselves as far apart as possible to minimize repulsion. This arrangement, you know, determines the electron geometry.
The molecular geometry, on the other hand, only considers the positions of the atoms themselves, not the lone pairs. It's a subtle but very important difference, you know, between the two geometries.
Electron Geometry of ClF2
For ClF2-, the central Chlorine atom has two bonding pairs (to the two Fluorine atoms) and three lone pairs. That's a total of five electron groups around the central atom. Five electron groups will arrange themselves in a trigonal bipyramidal electron geometry to minimize repulsion.
So, the electron geometry for ClF2- is trigonal bipyramidal. This shape is, like, a very common arrangement for five electron groups, so it's good to remember.
Molecular Geometry of ClF2
Now, to find the molecular geometry of ClF2-, we only look at the positions of the atoms. The central Chlorine has two Fluorine atoms bonded to it, and three lone pairs. In a trigonal bipyramidal arrangement, the lone pairs typically occupy the equatorial positions to minimize repulsion, especially with other lone pairs.
With the two bonding pairs at the axial positions and the three lone pairs in the equatorial plane, the resulting molecular geometry is linear. The F-Cl-F atoms line up perfectly straight, you know. It's a pretty interesting outcome for something with so many electron groups.
The ClF2 Puzzle: Hybridization and Polarity
Beyond just the shape, understanding the ClF2 Lewis structure also helps us figure out concepts like hybridization and polarity. These ideas explain even more about how a molecule behaves. So, they are, like, the next layer of understanding.
Hybridization tells us about the mixing of atomic orbitals to form new hybrid orbitals suitable for bonding. Polarity, on the other hand, describes how electrons are shared within the molecule. Both are pretty important for predicting chemical properties.
Understanding Hybridization
Hybridization is a theoretical concept that helps explain the observed molecular geometries. It involves the mixing of atomic orbitals (like s, p, and d orbitals) to form new hybrid orbitals that are, in a way, better suited for forming bonds. This concept helps explain why methane, for example, has four identical bonds.
The number of hybrid orbitals formed equals the number of atomic orbitals mixed. This number usually matches the number of electron groups around the central atom. It's a pretty neat trick that chemists use to make sense of bonding.
ClF2 Hybridization
For ClF2-, the central Chlorine atom has five electron groups (two bonding pairs and three lone pairs). According to VSEPR theory, these five electron groups arrange themselves in a trigonal bipyramidal electron geometry. To accommodate five electron groups, the central atom typically undergoes sp3d hybridization.
So, the hybridization of the central Chlorine atom in ClF2- is sp3d. This means one s orbital, three p orbitals, and one d orbital from Chlorine have mixed to form five equivalent sp3d hybrid orbitals. This is, you know, quite common for expanded octets.
Is ClF2 Polar?
To determine if ClF2- is polar, we need to consider both the polarity of its individual bonds and the overall molecular geometry. A molecule is polar if it has a net dipole moment, meaning there's an uneven distribution of electron density. This often happens when there's a difference in electronegativity between bonded atoms.
The Cl-F bonds are polar because Fluorine is more electronegative than Chlorine, pulling electron density towards itself. However, because the molecular geometry of ClF2- is linear, the bond dipoles cancel each other out. The three lone pairs on the central atom are arranged symmetrically around the linear F-Cl-F axis, too, so their influence also cancels out. Therefore, ClF2- is a nonpolar molecule. It's, like, a very important distinction to make.
Common Questions About ClF2 Lewis Structures
People often have a few questions when they first encounter the ClF2 Lewis structure. Let's address some of the most common ones. These questions, you know, often pop up in classrooms.
What is the formal charge on chlorine in ClF2?
As we calculated earlier, the formal charge on the central Chlorine atom in ClF2- is -1. This is because Chlorine has 7 valence electrons, 6 non-bonding electrons (from its three lone pairs), and 4 bonding electrons (from its two single bonds). So, 7 - 6 - (1/2 * 4) = -1. This negative formal charge, you know, matches the overall charge of the ion.
Does ClF2 follow the octet rule?
No, the central Chlorine atom in ClF2- does not follow the octet rule. It has 10 electrons around it (2 bonding pairs + 3 lone pairs = 5 electron groups, or 4 bonding electrons + 6 lone pair electrons = 10 total electrons). This is an example of an "expanded octet." Atoms in the third period and beyond, like Chlorine, can often accommodate more than eight valence electrons due to the availability of d orbitals. It's, like, a pretty common exception to the rule.
Why is Cl the central atom in ClF2?
Chlorine is the central atom in ClF2- for a couple of reasons. First, it is less electronegative than Fluorine. The least electronegative atom usually takes the central position. Second, Chlorine can form more than one bond and can expand its octet, allowing it to bond to two Fluorine atoms and still accommodate lone pairs. Fluorine, on the other hand, is very electronegative and typically forms only one bond. So, it's a pretty logical choice for the center.
Putting It All Together: Why This Matters for You
Learning about the ClF2 Lewis structure, its geometry, and its polarity is a really good step in understanding chemical bonding. These skills are, in a way,
Lewis Structure
Lewis Structure
N2 Lewis Structure - Chemistry Steps