NF3 Polar: A Closer Look At Molecular Polarity
Have you ever wondered why some chemical compounds seem to have a kind of internal compass, while others just sit there, well, balanced? It is a fascinating aspect of chemistry, one that shapes how these tiny bits of matter interact with everything around them. Today, we are going to talk about a specific compound, nitrogen trifluoride, often written as NF3, and explore its unique characteristics. We will find out if NF3 is polar, and what that even means for its behavior.
You see, understanding if a molecule has a "polar" nature helps us predict so much about it. It influences how a substance might dissolve in water, or how it might behave in various chemical reactions. For anyone curious about the unseen forces that shape our physical world, this idea of polarity offers some truly interesting insights. It is, in a way, about how electrons are shared, or not so much shared, between atoms.
We will unpack the details of NF3's structure and the elements it contains. As we learn from My text, NF3 is a covalent compound. It has one nitrogen atom joined to three fluorine atoms. This simple arrangement, actually, hides a surprising story about electron distribution. So, let's get into the specifics of what makes NF3 behave the way it does.
Table of Contents
- Understanding Molecular Polarity
- The Structure of NF3
- Why is NF3 Polar?
- Real-World Implications of NF3 Polarity
- Frequently Asked Questions About NF3 Polarity
Understanding Molecular Polarity
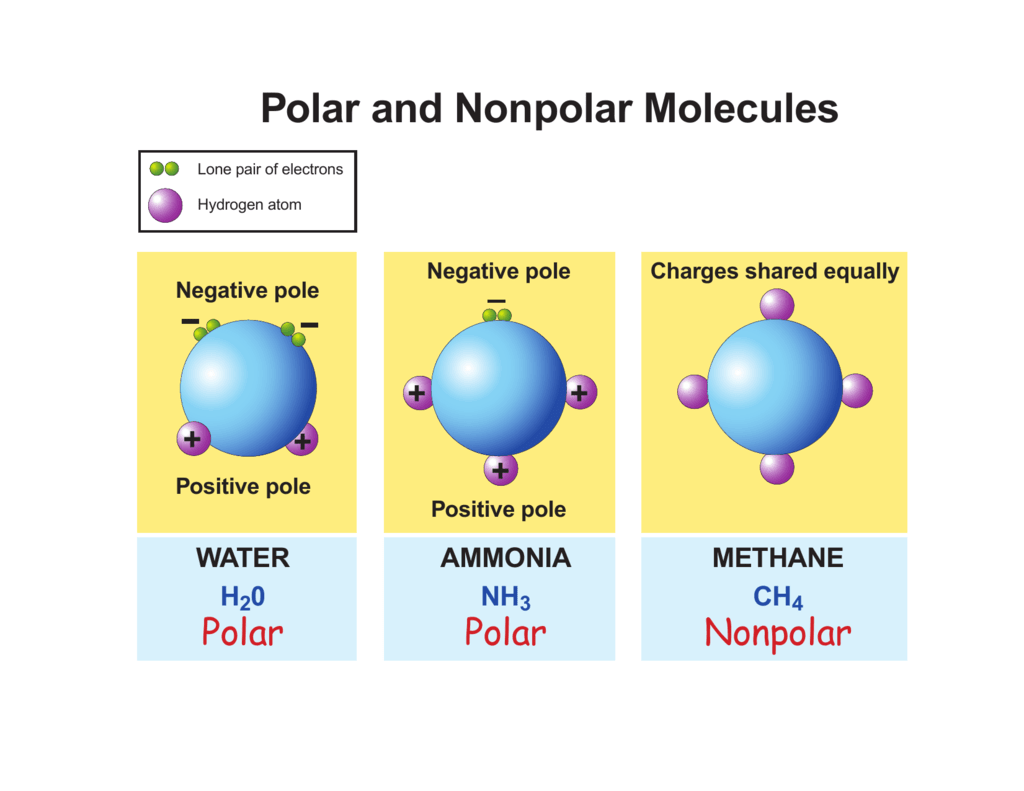
To really get why NF3 might be polar, we first need to grasp what molecular polarity truly means. It is, basically, about whether a molecule has an uneven distribution of electric charge. Think of it like a tiny magnet, with one end slightly positive and the other slightly negative. Not all molecules have this property, though. Some are perfectly balanced, with no overall charge separation. This balance, or lack of it, comes down to how atoms share their electrons.
A molecule's polarity, you see, is determined by two main things. One is the polarity of its individual bonds. The other is the overall shape of the molecule. Both of these parts work together to decide if a compound will have that slight positive and negative end. It is, quite literally, a balancing act of forces.
What is Electronegativity?
At the heart of bond polarity is something called electronegativity. This is, essentially, an atom's pull on shared electrons in a chemical bond. Imagine two friends trying to share a toy. If one friend is a bit stronger, they will pull the toy closer to themselves more often. In the same way, some atoms are better at pulling electrons towards their side of a bond. This pulling ability is what we call electronegativity. It is a very important concept in chemistry.
The periodic table, you know, gives us clues about this pulling power. Atoms closer to the top right, like fluorine, tend to have a much stronger pull. Those on the bottom left, like francium, have a weaker pull. This difference in pulling strength between two bonded atoms is what creates a polar bond. If the pull is strong enough, the electrons spend more time near the more electronegative atom, making that side slightly negative. The other side becomes slightly positive. So, a difference in electronegativity is key.
Covalent Bonds and Electron Sharing
When atoms share electrons, they form what we call covalent bonds. This is different from ionic bonds, where electrons are completely transferred. In a covalent bond, the electrons are, well, shared between the atoms. Yet, this sharing is not always equal, as we just discussed with electronegativity. Sometimes, the sharing is perfectly even, like two equally strong friends sharing a toy. This makes a nonpolar covalent bond.
Other times, one atom pulls the shared electrons a bit more strongly. This creates a polar covalent bond. The electrons spend more time near the atom with the stronger pull, giving it a partial negative charge. The atom that loses some of the electron density gets a partial positive charge. This partial charge separation is what makes the bond polar. It is a rather common occurrence in many compounds, as a matter of fact.
The Structure of NF3
Now, let's apply these ideas to NF3 itself. As My text explains, nitrogen trifluoride is a covalent compound. It consists of one nitrogen atom joined to three fluorine atoms. This arrangement might seem simple at first glance, but the way these atoms connect and the space they occupy around the central nitrogen atom is what really matters for its polarity. You see, the shape is not just a random thing; it is dictated by electron repulsion.
The central atom in NF3 is nitrogen. It is surrounded by three fluorine atoms. What is also important, though, is that the nitrogen atom also has a lone pair of electrons. These are electrons that are not involved in bonding with other atoms. This lone pair, quite literally, takes up space and influences the overall shape of the molecule. This spatial arrangement is what we need to picture.
Nitrogen and Fluorine Atoms
Consider the players here: nitrogen and fluorine. Nitrogen, which is in Group 15 of the periodic table, has five electrons in its outermost shell. Three of these electrons form bonds with the three fluorine atoms. This leaves nitrogen with one pair of electrons that are not involved in bonding. This unshared pair, sometimes called a lone pair, is quite significant for the molecule's shape and behavior. It is a bit like an extra "arm" that pushes other parts away.
Fluorine, on the other hand, is a highly reactive element from Group 17. It has seven electrons in its outermost shell. When it bonds with nitrogen, it shares one electron with nitrogen to complete its own electron shell. Fluorine is, actually, the most electronegative element on the periodic table. This means it has a very strong pull on shared electrons. This strong pull, too, is a critical part of the story for NF3's polarity.
The Lone Pair Effect
The presence of that lone pair on the nitrogen atom is, arguably, the biggest reason NF3 is polar. Electron pairs, whether they are bonding pairs or lone pairs, repel each other. They try to get as far away from each other as possible. This repulsion determines the molecule's geometry. With three bonding pairs and one lone pair around the central nitrogen, the molecule adopts a trigonal pyramidal shape. Think of a pyramid with the nitrogen at the top and the three fluorine atoms forming the base. The lone pair sits above the nitrogen, pushing the N-F bonds downwards. It is, in some respects, like a little invisible balloon pushing things around.
If there were no lone pair, and only three bonding pairs, the molecule would be flat, like a triangle. This flat shape would allow bond dipoles to cancel out. But because of the lone pair, the molecule is not flat. This non-symmetrical shape is what allows the individual bond polarities to add up, creating an overall molecular polarity. This is a very important distinction, as a matter of fact.
Why is NF3 Polar?
So, putting all these pieces together, why does NF3 end up being a polar molecule? It comes down to two main factors: the polar nature of its individual N-F bonds and the molecule's specific three-dimensional shape. Both of these must be present for a molecule to have an overall dipole moment, which is the measure of its polarity. If one of these parts is missing, the molecule might not be polar. It is, essentially, a combination of these two features.
Even though the N-F bonds are polar, if the molecule had a perfectly symmetrical shape, these polarities could cancel each other out. But with NF3, that is just not what happens. The lone pair on the nitrogen atom ensures the molecule is not symmetrical, preventing the cancellation of the bond dipoles. This is a key insight, you know, for understanding its behavior.
Bond Dipoles at Work
Let's look at the N-F bonds. Fluorine is much more electronegative than nitrogen. This means that in each N-F bond, the shared electrons are pulled more strongly towards the fluorine atom. This creates a partial negative charge on each fluorine atom and a partial positive charge on the nitrogen atom. Each N-F bond, therefore, has its own little dipole moment, pointing from the nitrogen towards the fluorine. It is, like your, tiny little arrows pointing towards the fluorine atoms.
These individual bond dipoles are like little vectors, pointing in specific directions. If you have three arrows pointing outwards from a central point, their combined effect depends on their angles. In a perfectly symmetrical arrangement, these arrows might pull equally in opposite directions, resulting in no net pull. But in NF3, the arrows do not perfectly oppose each other, allowing for a net effect. This is, basically, the crux of the matter.
Molecular Geometry Matters
The trigonal pyramidal shape of NF3 is what prevents the bond dipoles from canceling out. Imagine the nitrogen at the peak of a small pyramid, with the three fluorine atoms forming the base. The lone pair of electrons sits above the nitrogen, pushing the N-F bonds downwards. This arrangement means that the individual bond dipoles, which point from nitrogen towards each of the fluorine atoms, do not perfectly oppose each other. Instead, they all have a component that points downwards, away from the lone pair. This creates a net dipole moment for the entire molecule. So, the shape is, arguably, everything here.
If NF3 were, say, flat and symmetrical, like boron trifluoride (BF3), then even with polar B-F bonds, the molecule would be nonpolar because the dipoles would cancel. But the lone pair on nitrogen makes all the difference for NF3. It distorts the symmetry, allowing the individual bond polarities to add up. This net dipole moment means NF3 has a slightly negative end and a slightly positive end. This is what makes NF3 polar, as a matter of fact.
Real-World Implications of NF3 Polarity
The fact that NF3 is polar has some very real consequences for how it behaves. Polar molecules tend to dissolve well in other polar substances, like water. Nonpolar molecules, on the other hand, prefer to dissolve in nonpolar solvents, like oils. This "like dissolves like" principle is a fundamental rule in chemistry. So, knowing NF3 is polar helps us predict what it might mix with. It is, you know, a very practical piece of information.
Polarity also affects a molecule's boiling point and melting point. Polar molecules tend to stick together more strongly due to the attraction between their opposite partial charges. This means it takes more energy to separate them, leading to higher boiling and melting points compared to nonpolar molecules of similar size. This attraction, you see, is a bit like tiny magnets pulling on each other. These interactions, actually, influence many of a substance's physical properties. To learn more about how molecular forces shape everyday materials, you might want to explore resources like the American Chemical Society website, for instance.
Furthermore, polarity plays a part in how molecules react with each other. The positive end of one polar molecule might be attracted to the negative end of another, or to a charged ion. This can influence reaction pathways and rates. Understanding NF3's polarity is, therefore, quite important for chemists working with this compound. It helps them design experiments and predict outcomes. You can learn more about chemical bonding on our site, and link to this page exploring molecular shapes.
Frequently Asked Questions About NF3 Polarity
Here are some common questions people often have about NF3 and its polarity:
Is NF3 a polar molecule?
Yes, NF3 is, indeed, a polar molecule. This is because of the difference in electronegativity between nitrogen and fluorine, which creates polar N-F bonds. Also, the molecule has a trigonal pyramidal shape due to a lone pair of electrons on the nitrogen atom. This shape means the individual bond dipoles do not cancel each other out, leading to an overall net dipole moment for the molecule. So, it is polar.
What is the molecular geometry of NF3?
The molecular geometry of NF3 is trigonal pyramidal. This shape arises because the central nitrogen atom has three bonding pairs of electrons (with the three fluorine atoms) and one lone pair of electrons. These four electron groups arrange themselves in a way that minimizes repulsion, resulting in the pyramid-like structure. It is, basically, a very specific three-dimensional arrangement.
How does NF3 compare to NH3 in terms of polarity?
Both NF3 and NH3 (ammonia) are trigonal pyramidal and polar. However, there is a key difference in the direction of their overall dipole moments. In NH3, nitrogen is more electronegative than hydrogen, so the bond dipoles point towards the nitrogen, and they add up with the lone pair's contribution to point towards the nitrogen. In NF3, fluorine is more electronegative than nitrogen, so the bond dipoles point away from the nitrogen, towards the fluorines. The lone pair still contributes, but the overall dipole moment in NF3 points away from the lone pair and towards the fluorine atoms. So, they are both polar, but in a slightly different way, you know.
Solved Classify each molecule as polar or nonpolar. Polar | Chegg.com
Polar and Nonpolar Molecules
SOLVED: Are sulfur tetrafluoride (SF4) and nitrogen trifluoride (NF3